On Thursday, the Meals and Drug Administration decided that’s prone to have a serious affect on the panorama of Alzheimer’s illness analysis. The company issued a full-throated conventional approval of the drug Leqembi, developed by the businesses Eisai and Biogen. The drug is the primary of its class to obtain such approval and is meant to decelerate the development of the neurodegenerative illness.
Leqembi is one in all a number of antibody-based medicine that concentrate on amyloid beta, a protein that performs an essential function in Alzheimer’s. In these with the illness, a misfolded type of amyloid builds up within the mind over time, inflicting the event of hardy clumps referred to as plaques. These plaques, together with the buildup of one other misfolded protein referred to as tau, are thought to assist step by step destroy the mind. By breaking down or stopping the formation of plaques, it’s hoped that these medicine can cease or sluggish individuals’s cognitive decline.
In January 2023, the FDA issued an accelerated approval of Leqembi. Any such approval permits firms to solely current oblique proof that their drug will likely be clinically significant to sufferers—on this occasion, the discount of amyloid plaque. However firms are nonetheless required to gather knowledge and ultimately affirm a drug’s scientific advantages in an effort to obtain conventional approval. And it seems that Leqembi has now met that benchmark.
“At present’s motion is the primary verification {that a} drug focusing on the underlying illness technique of Alzheimer’s illness has proven scientific profit on this devastating illness,” mentioned Teresa Buracchio, performing director of the Workplace of Neuroscience within the FDA’s Middle for Drug Analysis and Analysis, in a assertion launched Thursday.
Within the pivotal 18-month-long scientific trial that secured Leqembi’s approval, the drug was discovered to sluggish the development of cognitive decline by 27% in sufferers in comparison with these on placebo. Sufferers additionally carried out higher on checks of their each day functioning and had decrease ranges of amyloid of their brains.
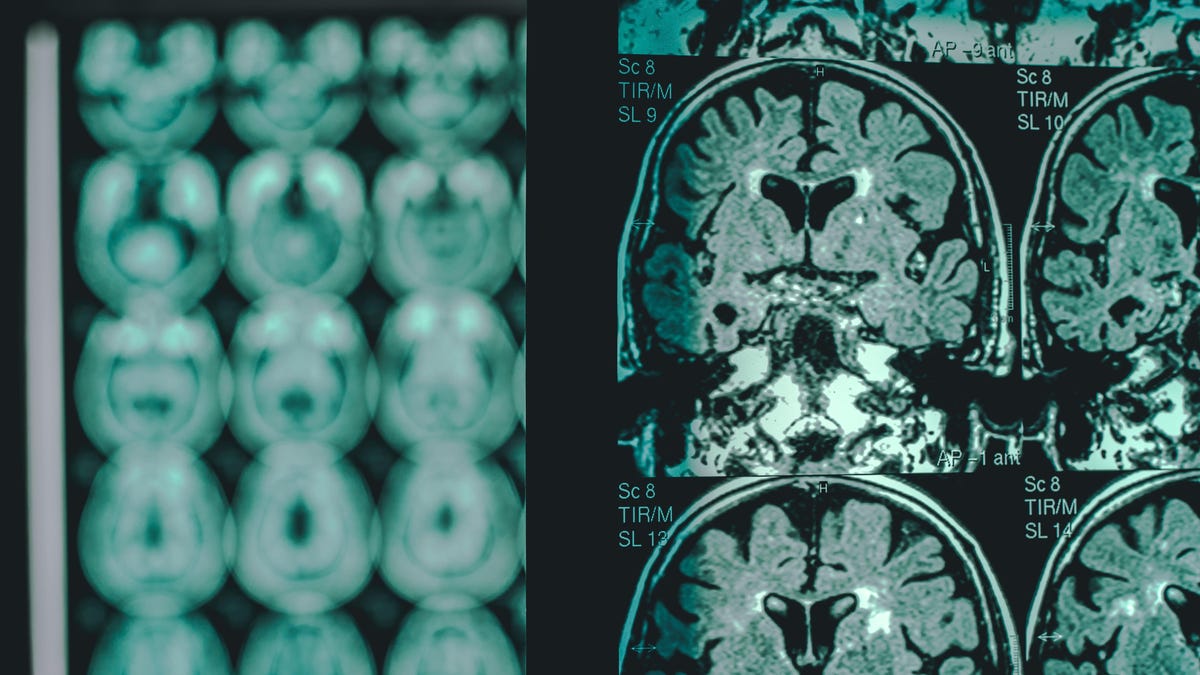
Anti-amyloid medicine aren’t with out their unintended effects, nonetheless. Some of the frequent issues is referred to as amyloid-related imaging abnormalities (ARIA), which might be identified through MRI. ARIAs are usually attributable to momentary swelling within the mind, however typically, they could be a signal of life-threatening bleeding. Most circumstances of ARIA resolve with out drawback, with many sufferers experiencing no signs, however there have been a number of deaths linked to ARIAs and these medicine.
The chance of ARIA and extreme ARIA appears to be greater in these carrying a selected Alzheimer-related mutation referred to as ApoE ε4. The usage of blood thinners may additionally be one other threat issue for extreme mind bleeding in these sufferers. Because of this, the drug’s labeling will name for docs to check sufferers’ ApoE ε4 standing earlier than prescribing it and can advocate added warning for contemplating its use in these taking blood thinners.
The standard approval of Leqembi will sidestep an argument surrounding these anti-amyloid medicine. In June 2021, the FDA issued an accelerated approval to the drug Aduhelm, additionally developed by Biogen and Eisai. The info supporting Aduhelm’s approval was decidedly weak and plenty of exterior specialists (together with a majority of these appointed by the FDA to advise the company) protested the choice. Ultimately, Medicare dominated that it could not routinely cowl Aduhelm and comparable medicine given accelerated approval till clear proof of its advantages was collected. The FDA was later harshly criticized by lawmakers for its “irregular” approval of the drug, and the drug’s makers have delayed plans to hunt approval elsewhere.
Although Aduhelm might by no means obtain full FDA approval and routine insurance coverage protection, the brand new Medicare coverage will now not apply to Leqembi. The drug’s present record worth ($26,000 a 12 months) can also be half as a lot because the preliminary worth of Aduhelm, one other issue that fueled widespread criticism of the latter. That mentioned, some researchers have continued to argue that Leqembi’s scientific advantages are possible too modest for sufferers and docs to be very enthusiastic about in the intervening time. However the drug class does seem like bettering. Earlier this Could, Eli Lilly’s donanemab offered the very best outcomes of its sort seen but, lowering individuals’s price of cognitive decline by 35% in comparison with a placebo in a large-scale trial.